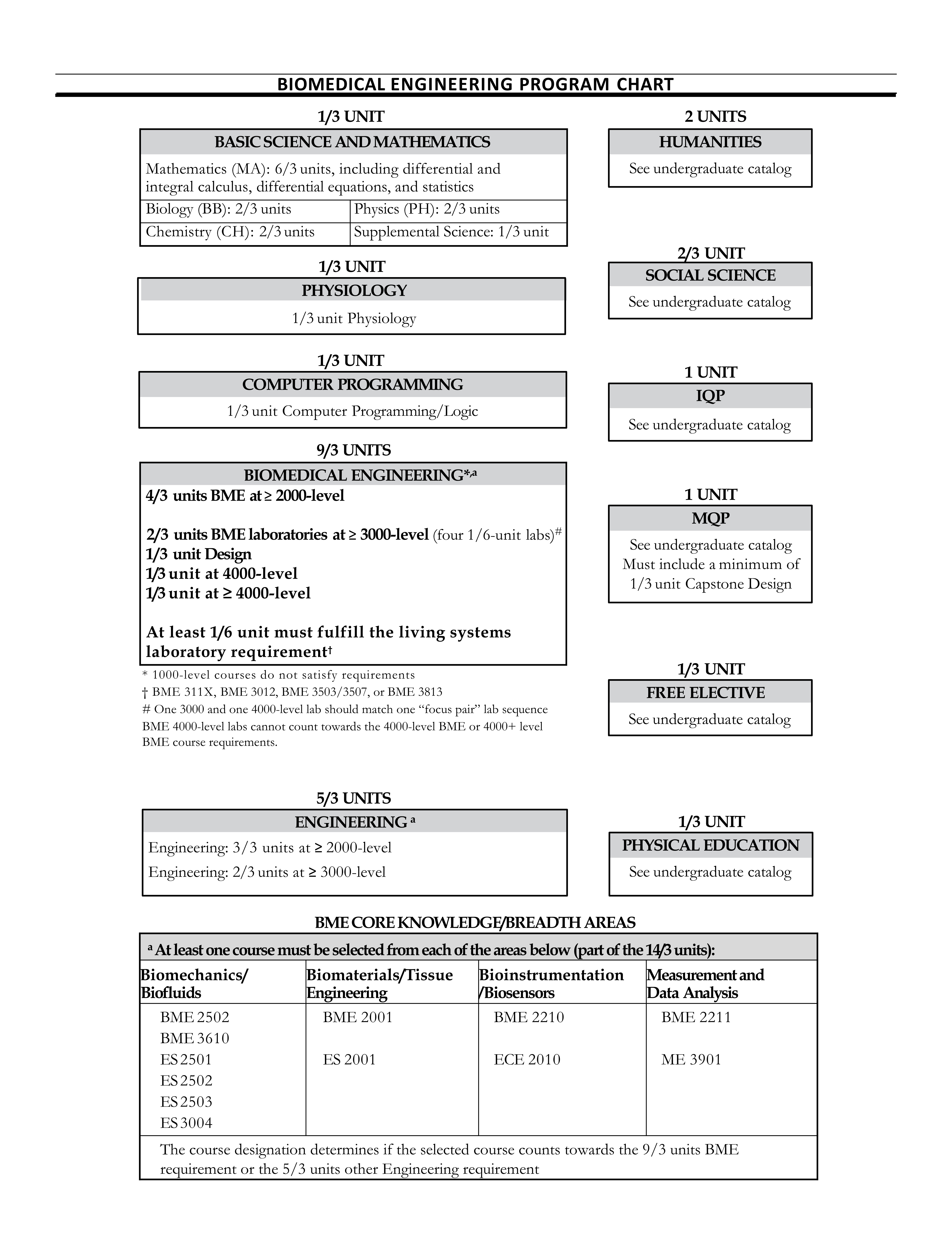
Program Distribution Requirements for the Biomedical Engineering Major
The normal period of residency at WPI is 16 terms. In addition to the WPI requirements applicable to all students, a biomedical engineer needs a solid background in mathematics, physical and life sciences. The distribution requirements are satisfied as follows.
Mathematics (Minimum 6/3 Units)
Mathematics must include differential and integral calculus, differential equations and statistics.
For the MA requirement, advanced courses within a-c may be allowed, but require department approval (via the course substitution form).
Basic Science (Minimum 6/3 Units)
Basic Science must include 2/3 unit from each of the following areas: BB, CH and PH. At least 1/3 unit of BB coursework must be 2000+ level.
Biology and Biochemistry
Chemistry and Biochemistry
Physics
Supplemental Science (Minimum 1/3 Units)
Supplemental Science must include 1/3 unit from BB, CH, CS, MA, PH, or FY courses that satisfy BB, CH, CS, MA or PH.
Biology and Biochemistry
Chemistry and Biochemistry
Computer Science
Mathematics
Physics
First Year Courses
FY courses must satisfy BB, CH, CS, MA or PH.
B. 2/3 unit of 3000+ level in Engineering
As part of the 14/3 units of engineering coursework, a subset of the courses must fulfill the following requirements:
No more than 1/3 unit of the 14/3 units of engineering coursework may be independent study (ISU) with a syllabus submitted to the Chair of the BME Undergraduate Curriculum Committee and a final report submitted to the ISU Instructor.
MQP credits cannot be used to satisfy the 14/3 units of engineering coursework.
For Requirements A-C above, you must take at least one course in each of the BME core competencies:
Major Qualifying Project (3/3 units)
Capstone Design Experience
Biomedical Engineering students must complete a Capstone Design experience requirement equivalent to 1/3 units of BME-related engineering design coursework. The Capstone Design experience may be fully accomplished by completing a BME design-based MQP that integrates prior course work and involves significant engineering design. At the time of registration for the MQP, the Project Advisor will determine whether the MQP will satisfy the 1/3 unit Capstone Design requirement. Alternatively, students who complete a BME research-based MQP as defined by the Project Advisor must take 1/3 unit of 4000-level BME engineering design coursework (BME 4301 or equivalent) to meet the ABET Capstone Design requirement. The MQP advisor is responsible for informing the student if the MQP satisfies Capstone Design requirements.
Biomedical Engineering Specializations
Because BME is such a broad and diverse discipline, it is convenient to subdivide it into a number of different specializations, or tracks. At the undergraduate level, these specializations help to bring focus to course and project planning. At the graduate-level, these specializations are aligned with the research interests of our faculty. Here at WPI, three specializations have been defined: 1) Biomechanics, 2) Biomedical Imaging and Instrumentation, and 3) Biomaterials and Tissue Engineering. If students are interested in developing an undergraduate program of study in one of these specializations, they should consult the Program of Study in BME sections of the catalog, within their chosen areas of specialization. See the department website for more details.
Biomechanics
Biomechanics is a specialization within biomedical engineering that involves the application of engineering mechanics to the study of biological tissues and physiological systems. When most people first think of biomechanics, the way we move or the strength of bones generally comes to mind. However, many other aspects are included in this diverse field of study including:
- Dynamics – e.g., analysis of human movement including walking, running, and throwing.
- Statics – e.g., determination of the magnitude and nature of forces in joints, bones, muscles and implanted prostheses, and characterization of the mechanical properties of the tissues in our bodies.
- Stress Analysis – e.g. calculation of the stresses and deformations within biological tissues and prostheses, and characterization of the mechanical properties of tissues and biomaterials.
- Fluid mechanics and transport – e.g., analysis flow of blood through arteries and air through the lung and diffusion of oxygen in tissues.
- Biomechanics research has improved our understanding of:
- Design and manufacturing of medical instruments, devices for disabled persons, artificial replacements, and implants.
- Human performance in the workplace and in athletic competition.
- Normal and pathological human and animal locomotion.
- The mechanical properties of hard and soft tissues.
- Neuromuscular control.
- The connection between blood flow and arteriosclerosis.
- Air flow and lung pathology.
- The effects of mechanical loads on cellular mechanics and physiology.
- Morphogenesis, growth, and healing.
- The mechanics of biomaterials.
- Engineering of living replacement tissue (tissue engineering)
Biomedical Instrumentation, Biosignals and Image Processing
Bioinstrumentation
Modern health care relies heavily on a large array of sophisticated medical instrumentation and sensors to diagnose health problems, to monitor patient condition and administer therapeutic treatments, most often in a non-invasive or minimally-invasive manner. During the past decade, computers have become an essential part of modern bioinstrumentation, from the microprocessor in a single-purpose wearable instrument used to achieve a variety of small tasks to more sophisticated desk-top instruments needed to process the large amount of clinical information acquired from patients. The Biomedical Instrumentation track of our program is focused on training students to design, test, and use sensors and biomedical instrumentation to further enhance the quality of health care. Emphasis is placed both on understanding the physiological systems involved in the generation of the measured variable or affected by therapeutic equipment, as well as the engineering principles of biomedical sensors and biomedical devices.
Examples of common biomedical instrumentations used routinely in medicine include:
- Specialized instrumentation for genetic testing.
- Electrocardiography to measure the electrical activity of the heart.
- Electroencephalography to measure the electrical activities of the brain.
- Electromyography to measure the electrical activities of muscles.
- Mechanical respirators.
- Cardiac pacemakers.
- Defibrillators.
- An artificial heart.
- Heart-lung machines.
- Pulse oximeters.
- Drug infusion and insulin pumps.
- Electrosurgical equipment.
- Anesthesia equipment.
- Kidney dialysis machines.
- Artificial electronic prosthetics used by disabled people (e.g. hearing aids).
- Laser systems for minimally invasive surgery.
Biosignals
Biosignal processing involves the collection and analysis of data from patients or experiments to identify and extract distinct components of the data set that may lead to better understanding of the processes involved in physiological regulation. For example, identifying and quantifying differences in the dynamic characteristics of physiological function between normal and diseased conditions utilizing biosignal processing techniques may lead to a better understanding of the role of regulatory imbalance in diseased conditions, and should have important clinical and diagnostic and prognostic application.
Examples of biosignal processing include:
- Detection of malignant heart rhythms from electrocardiograms.
- Early detection of sudden cardiac death.
- Monitoring of vital signs.
- Seizure detection using electroencephalogram recordings.
- Real-time control of artificial prosthetics.
- Real-time control of robotic movements.
- Early detection of hypertension and onset of diabetes.
- Wireless transmission of diagnostic devices.
- Modeling of pharmacokinetics and design of algorithms for robust drug delivery.
- Bioinformatics.
- Pattern recognition and decision support systems.
- Artificial intelligence.
Image Processing
Biomedical image processing involves the application of quantitative science and engineering to detect and visualize biological processes. An important area is the application of these tools to the study of diseases with an ultimate goal of aiding medical intervention. While x-ray imaging is an obvious and familiar example with tremendous diagnostic utility, it represents only a small aspect of this important field. Biomedical engineers are active participants in the development of new imaging modalities to acquire and process images from the body, most often in a non-invasive or minimally-invasive manner.
Examples of biomedical imaging and image processing include:
- X-ray imaging and computer-aided tomography (CAT).
- Visible light and optical imaging.
- Near-infrared imaging.
- Magnetic resonance imaging (MRI).
- Ultrasound imaging.
- Nuclear medicine imaging.
- Luminescence-based imaging.
Biomaterials and Tissue Engineering
Biomaterials
Biomaterials is a specialization within biomedical engineering that integrates engineering fundamentals in materials science with principles of cell biology, chemistry and physiology to aid in the design and development of materials used in the production of medical devices. When most people first think of biomaterials, implants such as surgical sutures, artificial hips or pacemakers generally come to mind, but many other aspects are included in this diverse field of study:
- Biomaterials Design – Identify the physiological and engineering criteria that an implantable biomaterial must meet. Select the proper chemical composition to insure that the biomaterial imparts the desired mechanical properties and evokes the appropriate tissue response for the specified application.
- Mechanics of Biomaterials – Characterize the magnitude and nature of the mechanical properties of biomaterials. Predict and measure how the physical/structural properties of a biomaterial determine its mechanical properties.
- Biomaterials-Tissue Interactions – Examine the molecular, cellular and tissue responses to implanted medical devices. Design biomaterials with properties that induce the desired wound healing and tissue remodeling responses from the body.
- Biomaterials research and development has improved our health care in many ways including:
- Design and manufacture of replacements parts for damaged or diseased tissues and organs (e.g., artificial hip joints, kidney dialysis machines)
- Improved wound healing (e.g., sutures, wound dressings)
- Enhanced performance of medical devices (e.g., contact lenses, pacemakers)
- Correct functional abnormalities (e.g., spinal rods)
- Correct cosmetic problems (e.g., reconstructive mammoplasty, chin augmentation)
- Aid in clinical diagnostics (e.g., probes and catheters)
- Aid in clinical treatments (e.g., cardiac stents, drains and catheters)
- Design biodegradable scaffolds for tissue engineering (e.g., dermal analogs)
Tissue Engineering
Tissue engineering integrates the principles and methods of engineering with the fundamentals of life sciences towards the development of biological substitutes to restore, maintain or improve tissue/organ function. When most people first think of tissue engineering, artificial skin and cartilage generally comes to mind, but many other aspects are included in this diverse field of study:
- Scaffold/Biomaterial Design – Identify the physiological and engineering criteria that a biodegradable scaffold must meet. Select the proper biochemical composition to insure that the cells perform in a physiologic manner on the surface of the scaffold.
- Functional/Biomechanical Tissue Engineering – Characterize the roles of biomechanical and biochemical stimuli on the formation, growth, development and function of bioengineered cells, tissues and organs. Create accurate biomimetic engineered tissue models of human disease to aid in the discovery, invention and development of novel therapeutic strategies.
- Bioreactor Design – Design reactors that control the rates at which nutrients and growth factors are supplied to bioengineered tissues and organs during growth and development in a laboratory environment.